KeriMedical
Reaxon®
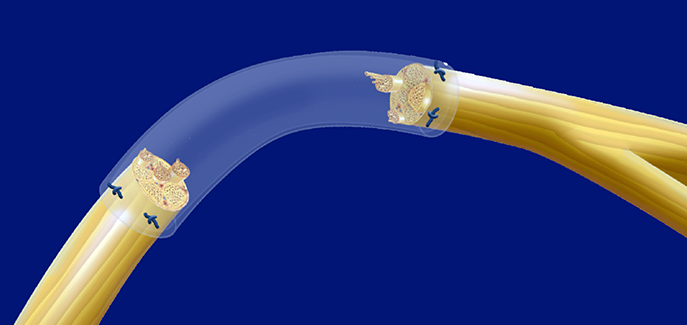
The only CHITOSAN based Nerve Regrowth and Protection Tube.
- Flexible and transparent
- Does not collapse
- Easy to suture (hydrogel)
- Consist of polysaccharides
WHAT IS CHITOSAN?
- Chitosan is a cationic polysaccharide of natural origin obtained by alkaline deacetylation of chitin.
- The chitin used in the production of Reaxon® tubes is of natural origin.
Chitosan tubes are:
- Biodegradable – they degrade to compounds that can be metabolized by the body1
- Bioactive – they stimulate the growth of nerve cells2
- Biocompatible – they do not induce any immune response3
- Antiadherent – they limit the formation of scar tissue4
- Antifungal, Antibacterial5
- Hemostatic1
ADVANTAGES OF CHITOSAN IN THE USE OF REAXON® TUBES
- The transparency of the tubes enables effective positioning of the fascicles and early detection of blood clots.
- Thanks to the mechanical properties of the tubes, suturing is facilitated (even with 9-0 sutures).
- Very low risk of an immune response: non-protein origin; not of porcine or bovine origin.
- Bioactive:
- Good tube integrity for 18 months (resorption at 2.5 years)2
- Nerve repair without tension to enhance healing and function recovery.
ADVANTAGES OF REAXON®
- No morbidity or loss of feeling at the donor site.
- Does not collapse.
- Highly transparent enabling optimal positioning of the nerve endings and detection of blood clots.
- Semi-permeable enabling small nutrients and neurotropic factors to cross the tube.
- The metabolic degradation products are resorbed via normal metabolic pathways.
- Good tube integrity for 18 months (resorption at 2.5 years).
Two versions are available:
/ REAXON® NERVE GUIDE
Assists nerve regrowth when a direct suture is not possible (for sutures with a loss of substance of up to 26 mm)
| Catalog Number | Inner Diameter | Lenght |
|---|---|---|
| RG321 | 2.1 mm | 30 mm |
| RG330 | 3.0 mm | 30 mm |
| RG340 | 4.0 mm | 30 mm |
| RG350 | 5.0 mm | 30 mm |
| RG360 | 6.0 mm | 30 mm |
/ REAXON® DIRECT
Enables protection when there is little or no loss of matter
| Catalog Number | Inner Diameter | Lenght |
|---|---|---|
| RD121 | 2.1 mm | 14 mm |
| RD130 | 3.0 mm | 14 mm |
| RD140 | 4.0 mm | 14 mm |
| RD150 | 5.0 mm | 14 mm |
| RD160 | 6.0 mm | 14 mm |
Product presentation
Publications and research
Clinical Trials Medovent
Enhancing the Outcome of Traumatic Sensory Nreve Lesions of the Hand by Additional Use of a Chitosan Nerve Tube in Primary Nerve Repair: A Randomized Controlled Bicentric Trial
Chitosan tubes of varying degrees of acetylation for bridging peripheral nerve defects
References:
- Croisier F, Jérôme C. Chitosan-based biomaterials for tissue engineering. European Polymer Journal. avr 2013;49(4):780-92.
- Haastert-Talini et al. Chitosan tubes of varying degrees of acetylation for bridging peripheral nerve defects. Biomaterials 2013; 34: 9886-9904.
- The biocompatibility of Reaxon® Nerve Guide has been proven in accordance with the ISO 10993 standards.
- Marcol et al. Reduction of post-traumatic neuroma and epineural scar formation in rat sciatic nerve by application of microcrystallic chitosan. Microsurgery 2011; 31: 642-649.
- Rabea et al. Chitosan as antimicrobial agent: application and mode of action. Biomacromolecules 2003; 4: 1457-1465.
- Oliveira et al. Chitosan drives anti-inflammatory macrophage polarisation and pro-inflammatory denditric cell stimulation. Eur Cell Mater 2012; 24: 136-52
- Yuan et al. The interaction of Schwann cells with chitosan membranes and fibers in vitro. Biomaterials 2004; 25: 4273-4278
- Freier et al. Chitin-based tubes for tissue engineering in the nervous system. Biomaterials 2005; 26: 6424-6432.